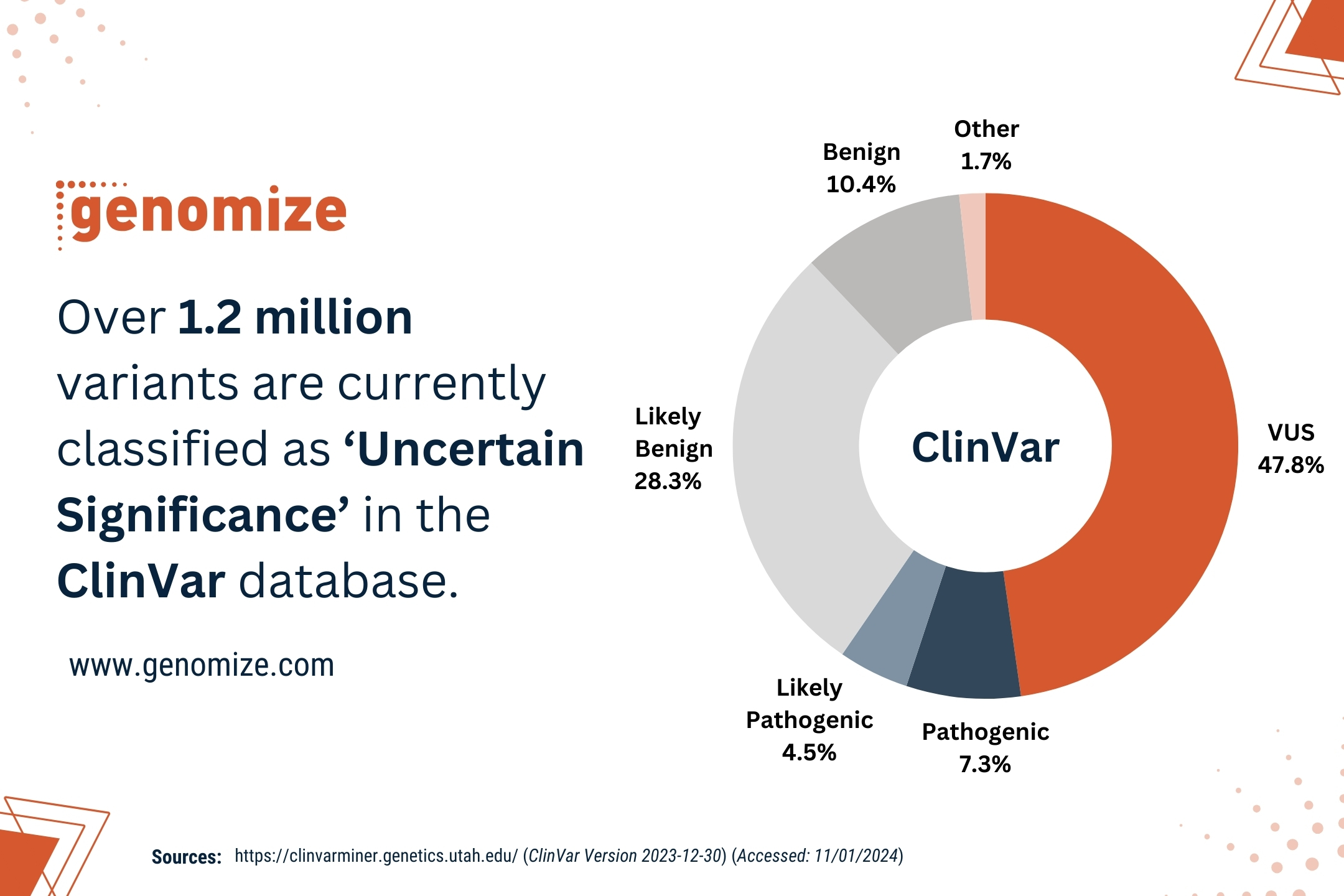
Navigating the Uncharted Territories: Are Rare Diseases Truly Rare?
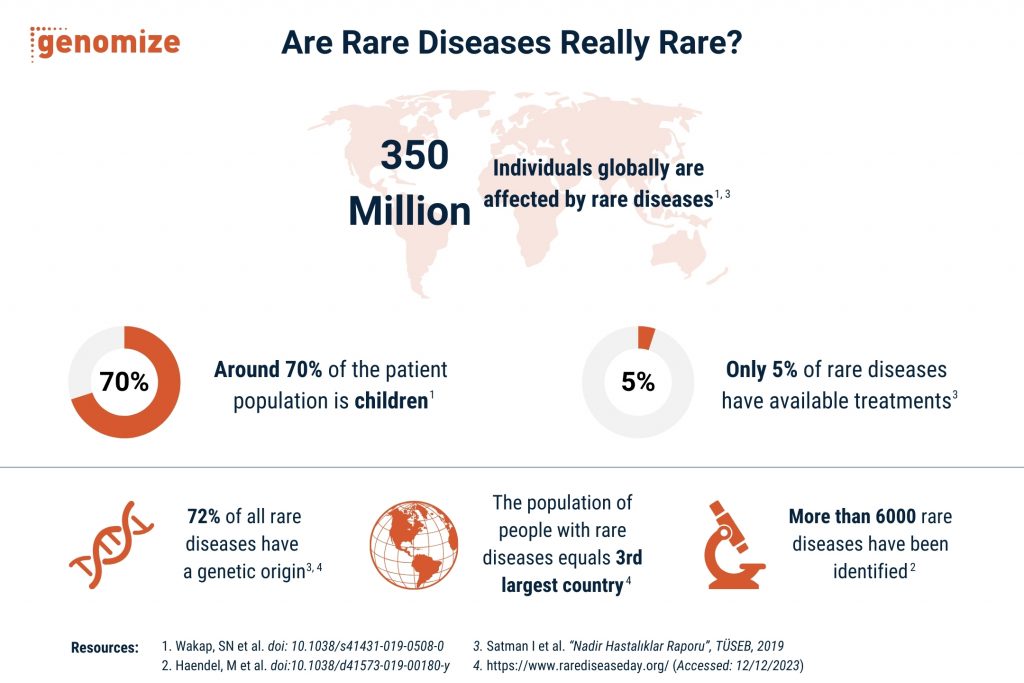
Rare diseases are chronic, progressive disorders that affect approximately 6-8% of the general population. About 70–80% of these diseases have a genetic origin [3, 4] and around 70% of the patient population is made up of children [1]. The prevalence of rare diseases worldwide affects between 5 to 60 individuals per 100,000 population (average 40/100,000). Therefore, an estimated 350 million individuals globally are affected by rare diseases and the frequency of these diseases increases proportionately with the number of consanguineous marriages [1, 3].
Although each rare disease impacts a relatively small number of individuals, the cumulative effect is significant because a large number of people are affected by rare diseases collectively. The main reason for this contradiction is the vast diversity of recognized rare diseases [1-5]. To understand the diversity of them, the effort to harmonize disease definitions in the Monarch Disease Ontology estimates over 10,000 rare diseases by collecting data from various sources such as Orphanet, OMIM, GARD, DOID, and NCI Thesaurus, with 6,370 diseases present in multiple resources [2]. Therefore, we can say that while each disease may appear rare on its own, the sheer volume and diversity of these conditions highlight their substantial cumulative impact.
The Diagnostic Odyssey
The diagnostic odyssey, the challenge to identify rare diseases because of the vague symptoms that overlap with more common disorders, further blurs the lines of the rarity of these diseases. Many individuals with these conditions endure an everlasting journey of medical consultations, complex diagnoses, and frustration before arriving at an accurate diagnosis. This endless process not only increases the physical and emotional toll on patients but can also lead to irreversible damage, as timely intervention is crucial for many rare conditions. Moreover, this delay in diagnosing a orphan disease further reinforces the misconception that these diseases are infrequent in medical practice [2].
Research across 17 European countries revealed that 25% of rare disease patients experienced delays ranging from 5 to 30 years before correct identification [6]. To address this challenge, efforts are directed towards developing genetic-based diagnostic tools to support clinicians and shorten this diagnostic journey. As most of these diseases stem from gene mutations, identifying these mutated genes is crucial for diagnosis. Advanced techniques like Next-Generation Sequencing (NGS), such as Whole Exome Sequencing (WES), offer swift, potent, and cost-effective genetic analyses, significantly enhancing the diagnostic success rate for rare diseases [7]. However, more than half of these diseases lack known causative genomic variants, leaving many patients undiagnosed due to this substantial gap in genetic understanding [8].
Conclusion and Genomize
Only 5% of rare diseases have available treatments, leaving the vast majority, termed ‘orphan diseases’, without viable options [3]. Genomize understands this paradox—while individually rare, these conditions collectively form a substantial and diverse group that demands attention. We believe in redefining the narrative surrounding rare diseases by offering analyses for comprehensive genetic diagnostic tests tailored to patients with complex medical histories or symptoms hinting at genetic disorders. With our cutting-edge Next Generation Sequencing (NGS) data analysis software, the SEQ Platform, we navigate the uncharted territories of rare diseases, providing hope for accurate and swift diagnoses.
The SEQ platform is integrated with an AI-driven Variant Prioritization tool, receiving data from 120 different data sources to match phenotype-gene-disease information from the databases and the literature with 97% accuracy [9]. You can read our technical white paper to get more information about our AI algorithm. This variant prioritization tool rapidly produces a shortlist of clinically relevant genetic variants that could explain the clinical symptoms of the patient and reduce the time for variant analysis. Thus, our AI-assisted variant prioritization algorithm improves the accuracy of diagnosis and minimizes the likelihood of overlooking genetic variants.
In summary, Genomize’s SEQ Platform, with its AI-driven Variant Prioritization tool, combines advanced genetic testing with precise data analysis and offers quicker, more accurate diagnoses for rare diseases, and paving the way for personalized treatments.
References
1. Wakap, SN et al. Estimating cumulative point prevalence of rare diseases: analysis of the Orphanet database. Eur. J. Hum. Genet. Vol. 28,2 (2020):165-173. doi: 10.1038/s41431-019-0508-0.
2. Haendel, M et al. “How many rare diseases are there?.” Nature Reviews Drug Discovery vol. 19,2 (2020): 77-78. doi:10.1038/d41573-019-00180-y
3. Satman I et al. “Nadir Hastalıklar Raporu”, TÜSEB Türkiye Halk Sağlığı ve Kronik Hastalıklar Enstitüsü, 2019.
4. https://www.genome.gov/FAQ/Rare-Diseases (Accessed: 12/12/2023)
5. Ferreira, CR. The burden of rare diseases. Am J Med Genet Part A. vol. 179A (2019): 885–892. https://doi.org/10.1002/ajmg.a.61124
6. Sanges, S et al. “Raising rare disease awareness using red flags, role play simulation and patient educators: results of a novel educational workshop on Raynaud phenomenon and systemic sclerosis.” Orphanet journal of rare diseases vol. 15,1 (2020):159. doi:10.1186/s13023-020-01439-z
7. Fernandez-Marmiesse, A et al. “NGS Technologies as a Turning Point in Rare Disease Research, Diagnosis and Treatment.” Current medicinal chemistry vol. 25,3 (2018): 404-432. doi:10.2174/0929867324666170718101946
8. Turro, E et al. “Whole-genome sequencing of patients with rare diseases in a national health system.” Nature vol. 583,7814 (2020): 96-102. doi:10.1038/s41586-020-2434-2
9. https://genomize.com/ai-driven-ngs-data-analysis-a-variant-prioritization-pipeline-for-precision-medicine/ (Accessed: 12/12/2023)